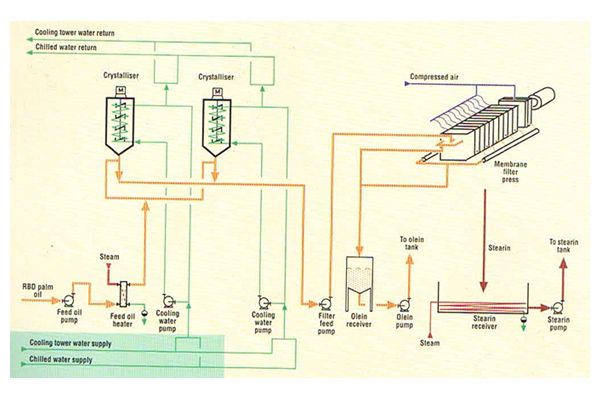
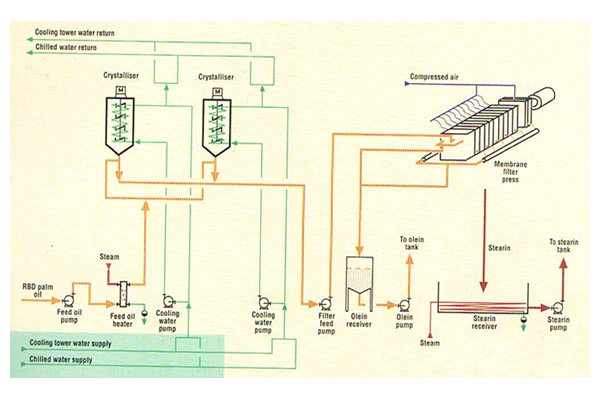
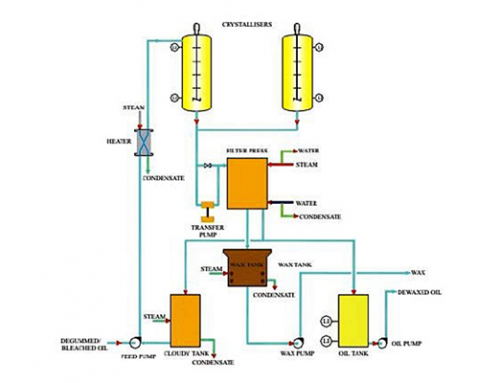
Dry Fractionation
OIL & FATS
Product Overview
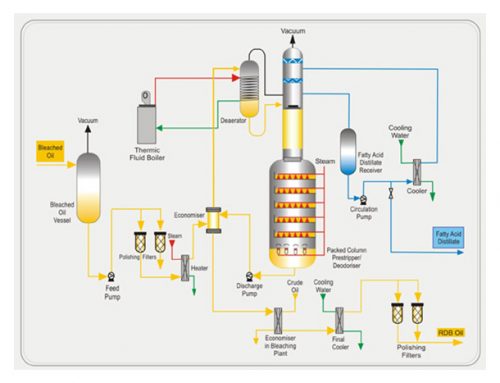
The widespread use of the three oil modification processes – hydrogenation, inter-esterification and fractionation – extended the range of applications of triglyceride oils. These processes principally serve the purpose of modifying the melting properties of oils and fats in order to improve their functional properties in specific applications, but the processes are also used to improve the stability of the oils and fats thus processed.
In edible oil processing, a fractionation process consists of controlled cooling of the oil, thereby inducing a partial, or ‘fractional’, crystallization. The remaining liquid (olein) is then separated from the solid fraction (stearin) by means of filtration or centrifugation. Natural oils and fats have different characteristics due to the fact that they are composed of a great number of different triglycerides. These contain fatty acids with carbon chains of different lengths and with different degrees of unsaturation.
Triglycerides with a high degree of unsaturation, indicated by a high iodine value, have a lower melting point than those containing more saturated fatty acids. If oil is cooled to a certain temperature, the high melting triglyceride (Stearin) will crystallize while the low melting ones will remain fluid. The stearin can then be separated from oil (Olein) by different methods, and the fat/oil is thus divided into two fractions: Stearin with a high melting point and olein with a low cloud and melting point. This technique is called fractional crystallization and is used to obtain oils or fats more suitable, for example, as cooking oils or for margarine/shortening production.
Three palm oil fractionation processes are in use:
- Dry Fractionation:
Through batch crystallization of oil without using additives by controlled cooling and subsequent continuous filtration.
- Solvent Fractionation:
Through continuous crystallization of the oil in a solvent followed by separation of the liquid and solid fractions through a continuous drum filter. Solvent fractionation involves using hexane or acetone to let the high-melting components crystallize in a very low-viscous organic solvent. This can be helpful with respect to the selectivity of the reaction but mainly offers advantages in the field of phase separation: much purer solid fractions can be obtained, even with vacuum filtration. Being a more expensive process, it is less common than dry fractionation and only comes into the picture when a very high added value of (at least one of) the resulting fractions makes up for the high cost.
- Detergent Fractionation:
Through batch or continuous crystallization of the oil by controlled cooling and separation of the fractions either by gravity or centrifugation after adding a surfactant.
Capacity
25 TPD to 500 TPD



Leave A Comment